UNIVERSITY OF KOBLENZ
Universitätsstraße 1
56070 Koblenz

Cutin and suberin are naturally occurring polyesters that form an outer barrier in plants between the plant and the surrounding atmosphere (cutin) or soil (suberin). Among other things, they therefore regulate the water balance of plants and provide protection against the penetration of plant pathogens. Each plant species has an individual pattern of components of these polyesters, which consist of fatty acids, long-chain dicarboxylic acids or hydroxycarboxylic acids, long-chain alkanols and alkanediols as well as glycerol. We synthesize selective isotope-labeled (2H, 13C) derivatives to investigate the biochemical degradation pathways of these compounds together with cooperation partners [1-3].
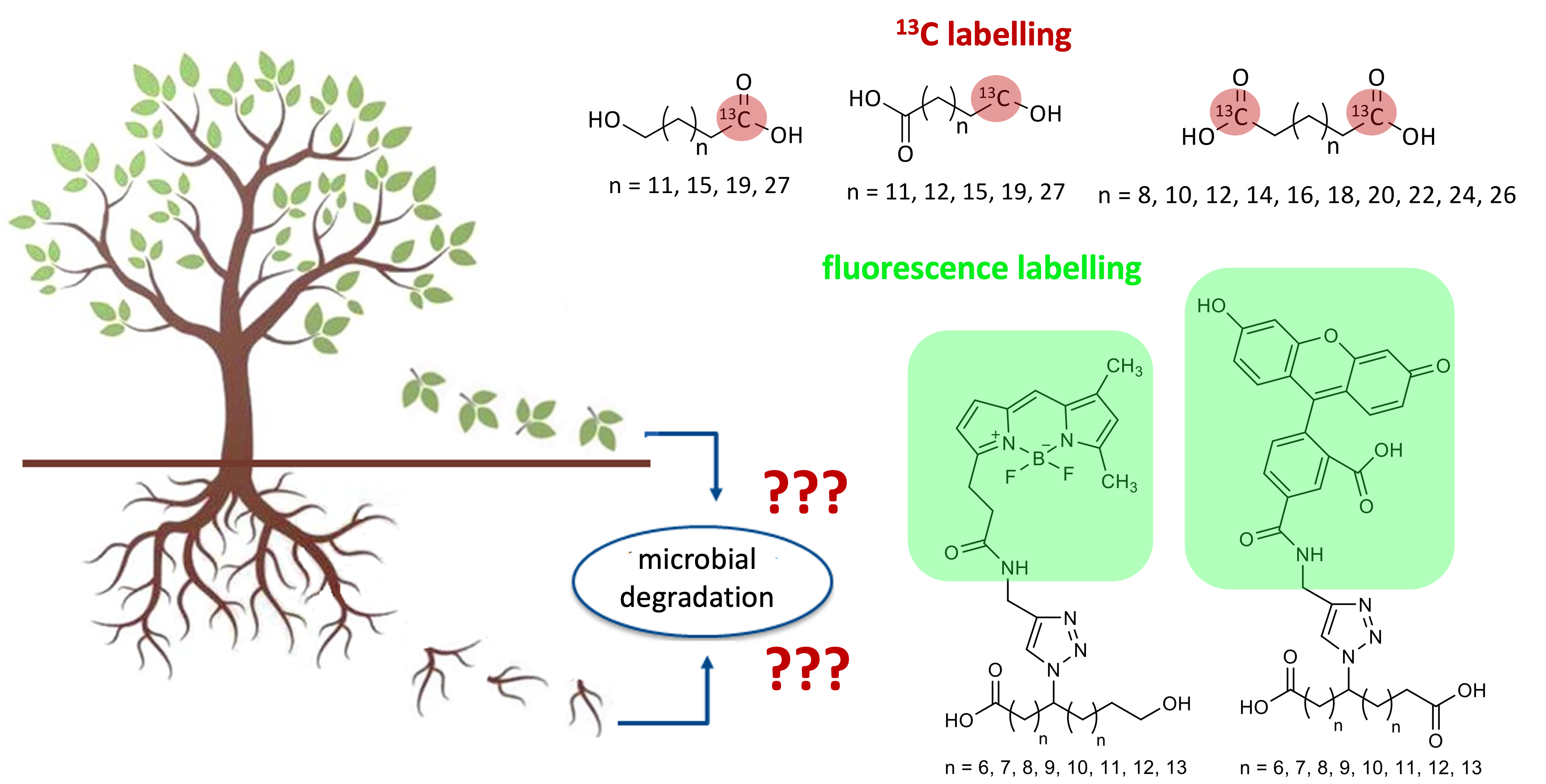
[1] Schink, C., Spielvogel, S., Imhof, W. (2021) Synthesis and characterization of 13C-labelled cutin and suberin monomeric dicarboxylic acids of the general formula HO213C-(CH2)n-13CO2H (n = 10, 12, 14, 16, 18, 20, 22, 24, 26, 28). J. Label. Compd. Radiopharm. 64, 14.
[2] Schink, C., Spielvogel, S., Imhof, W. (2021) Synthesis 13C-labelled ω-hydroxy carboxylic acids of the general formula HO213C-(CH2)n-CH2OH or HO2C-(CH2)n-13CH2OH (n = 12, 16, 20, 28). J. Label. Compd. Radiopharm. 64, 385.
[3] Kashi, H., Loeppmann, S., Herschbach, J., Schink, C., Imhof, W., Kouchaksaraee, R.M., Dippold, M.A., Spielvogel, S. (2022) Size matters: Biochemical mineralization and microbial incorporation of dicarboxylic acids in soil. Biogeochemistry 162, 79.
Microplastics (MP) are an increasingly significant problem for the environment and are now present globally. To understand the distribution and impact of MP on the environment, it is important to identify and quantify MP over a wide size range. Until now, microplastic particles (MPP) in environmental samples have mainly been identified and quantified by FTIR or Raman spectroscopy or by e.g. pyrolysis-GC/MS methods. In recent years, we have been able to establish quantitative NMR spectroscopy as a further method that allows size-independent, rapid and simple quantitative analysis of MPP and makes it possible to specify a concentration of MP in environmental samples, for example in mg MP per g environmental sample [1-7].

[1] Peez, N., Janiska, M.-C., Imhof, W. (2019) The first application of quantitative 1H-NMR-Spectroscopy as a simple and fast method of identification and quantification of microplastic particles (PE, PET and PS). Anal. Bioanal. Chem. 441, 823.
[2] Peez, N., Becker, J., Ehlers, S., Fritz, M., Fischer, C.B., Koop, J.H.E., Winkelmann, C., Imhof, W. (2019) Quantitative analysis of PET microplastics in environmental model samples using quantitative NMR spectroscopy: Validation of an optimized and consistent clean-up method. Anal. Bioanal. Chem. 441, 7409.
[3] Peez, N., Imhof, W. (2020) Quantitative 1H-NMR spectroscopy as an efficient method for identification and quantification of PVC, ABS and PA microparticles. Analyst 145, 5363.
[4] Peez, N., Rinesch, T., Kolz, J., Imhof, W. (2021) Applicable and cost-efficient microplastic analysis by quantitative 1H-NMR spectroscopy using benchtop NMR and NoD methods. Magn. Res. Chem. 60, 172.
[5] Günther, M., Imhof, W. (2023) Simultaneous quantification of microplastic particles by NoD 1H-qNMR from samples comprising different polymer types. Analyst 148, 1151.
[6] Seghers, J., Günther, M., Breidbach, A., Peez, N., Imhof, W., Emteborg, H. (2023) Feasibility of using quantitative 1H-NMR spectroscopy and ultra-micro balances for investigation of a PET microplastics reference material. Anal. Bioanal. Chem. 415, 3033.
[7] Günther, M., Imhof, W. (2024) Highly selective solid liquid extraction of microplastic mixtures as a pre-preparation tool for quantitative nuclear magnetic resonance spectroscopy studies. Analyst 149, online ahead of print.
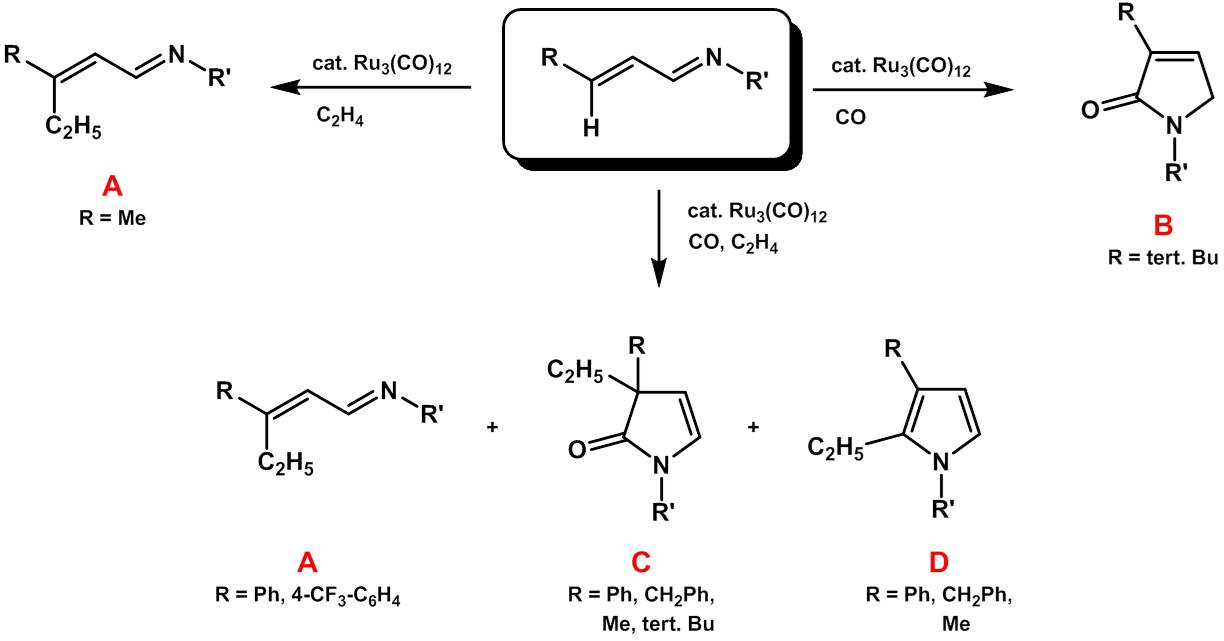
Unsaturated imines can be reacted with CO and alkenes (in the simplest case ethylene) to form heterocyclic compounds. Type A and B compounds are formed in reactions in which only either carbon monoxide or ethylene is present. In reactions of both substrates, the 1,3-dihydropyrrolone C is usually formed in almost quantitative yield if a non-polar solvent is used. The proportion of 2,3-disubstituted pyrroles, D, increases with the polarity of the solvent used. It is also possible to further simplify the reaction in that it is not necessary to isolate the imine as a starting material, but that the reaction of an unsaturated aldehyde with a primary amine as the basic constituents of the imine with CO and ethylene leads to the same basic structures A-D. Using this four-component reaction, a high degree of complexity can therefore be generated in a single reaction step from commercially available starting materials, with the only waste product being one molecule of water per product molecule.
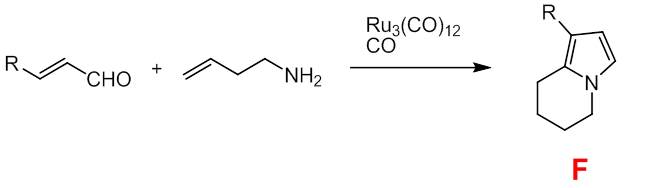
Syntheses in which the alkenyl and amino functions are present in the same substrate molecule should therefore be suitable for the simple construction of bicyclic systems. In the case of but-3-en-1-amine, this possibility could actually be realized for a series of residues R. In these reactions, only compounds of type F are selectively formed, but no lactam derivatives corresponding to B or C.
Current investigations are focusing on expanding the possible range of substrates, developing further tandem reactions made possible by the presence of additional functional groups, and elucidating the catalysis mechanism using DFT calculations.
We are grateful for the support of projects in connection with this area of research:

In collaboration with the Physics Department of the Institute of Integrated Natural Sciences (Prof. (UM6P) Dr. Christian B. Fischer), we have investigated the possibilities of DLC coatings (diamond like carbon) are coatings that can be applied to different substrates using a low-temperature plasma process. In contrast to diamond, however, the layers do not only consist of sp3-hybridized carbon atoms, but also contain a proportion of sp2-hybridized carbon centers that can be controlled to a certain extent by the deposition conditions. We want to transfer the already described bonding of organic groups by radical reaction of azo compounds with the surface to ligand fragments capable of coordination or ligands already coordinated in advance [1].
We would like to thank the Chemical Industry Fund for supporting this project.
[1] Schink, C., Catena, A., Heintz, K., Görls, H., Beresko, C., Ankerhold, G., von der Au, M., Meermann, B., Van Malderen, S.J.M., Vanhaecke, F., Imhof, W., Fischer, C.B. (2019) Attaching photochemically active Ruthenium polypyridyl complex units to amorphous hydrogenated carbon (a-C:H) layers. Adv. Mater. Interfaces 6, 1801308.
[2] Ghabri, W., Schlebrowski, T., Imhof, W., Fischer, C.R. (2022) Synchrotron-based Spectroscopic Study of Plasma Generated Amorphous Hydrogenated Carbon films (a-C:H) Post-Functionalized Using Photochemically Active Ruthenium-Polypyridyl Complexes. J. Electron Spectros. Relat. Phenomena 257, 147204.
[3] Ghabri, W., Imhof, W. (2024) Optimization of the synthesis, spectroscopic characterization and computational study of 4,4"-Azobis(2,2'-bipyridine). Eur. J. Org. Chem. 27, in preparation.