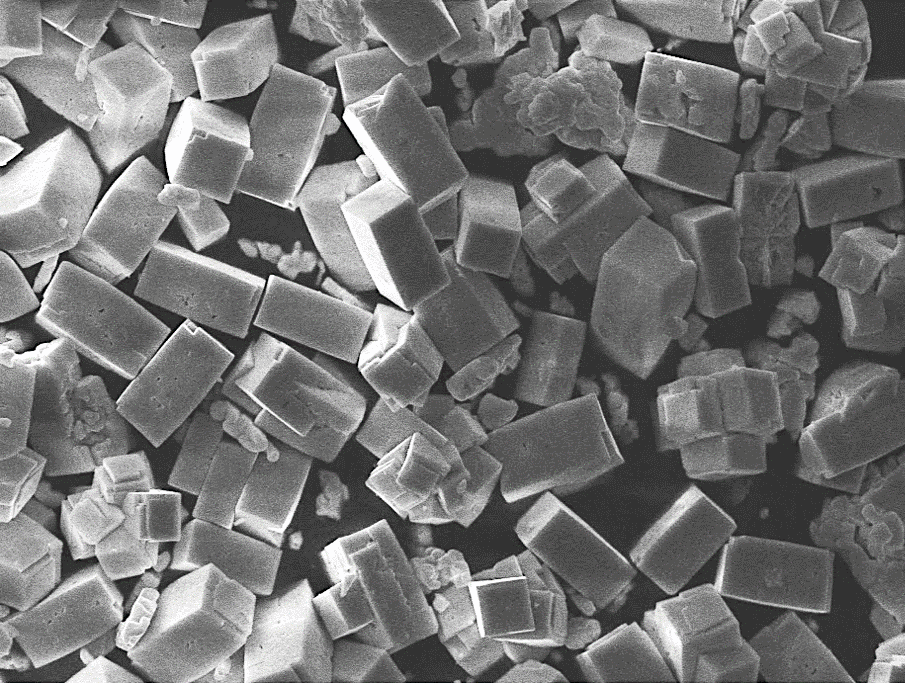The enhancement of the surface-to-volume ratio and the shortening of the diffusion path length of ionic and electronic charge carriers play a key role for the development of ceramic materials for energy conversion applications. For this reason, the preparation of nanostructured ceramics is targeted.
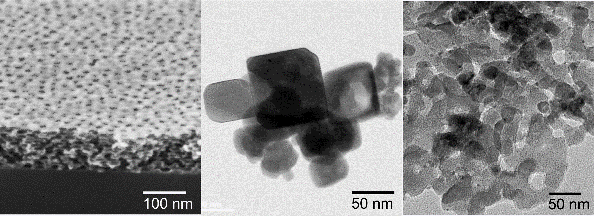
Nanostructured perovskite oxides are developed using colloidal synthesis approaches in form of powders, thin films and nanoparticles (Figure 1). Particular focus lies in the preparation of nanoporous structures with tunable specific surface area and pore size using different strategies with and without templating agents.
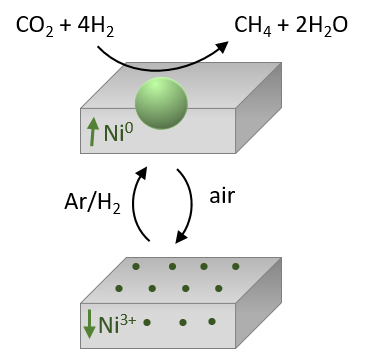
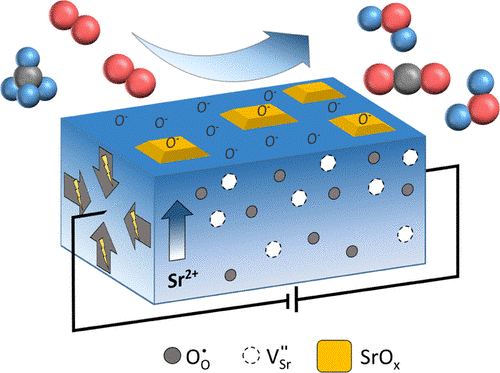
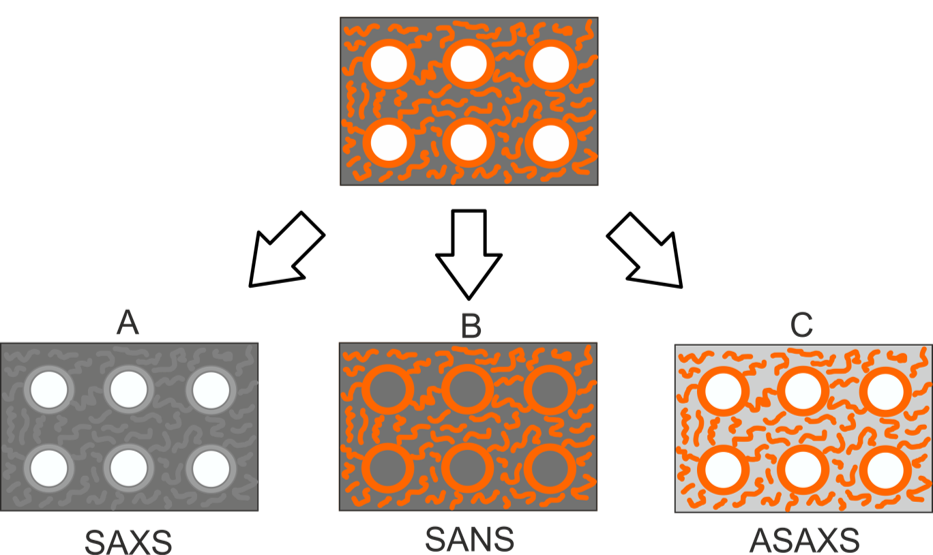
In order to exploit at best nanoporous perovskites for thermal catalysis and energy conversion applications the transport of ionic charge carriers (e.g. oxide ions and protons) in these structures needs to be carefully addressed. The unique combination of in situ X-ray diffraction with thermogravimetric experiments and mass spectroscopy allow us to understand the stong interdependency between ionic mobility and structural changes in the materials. Finally, high-temperature catalysis tests are carried out to correlate the catalytic performance with the structure-function relationships of the developed nanoporous perovskite oxides.